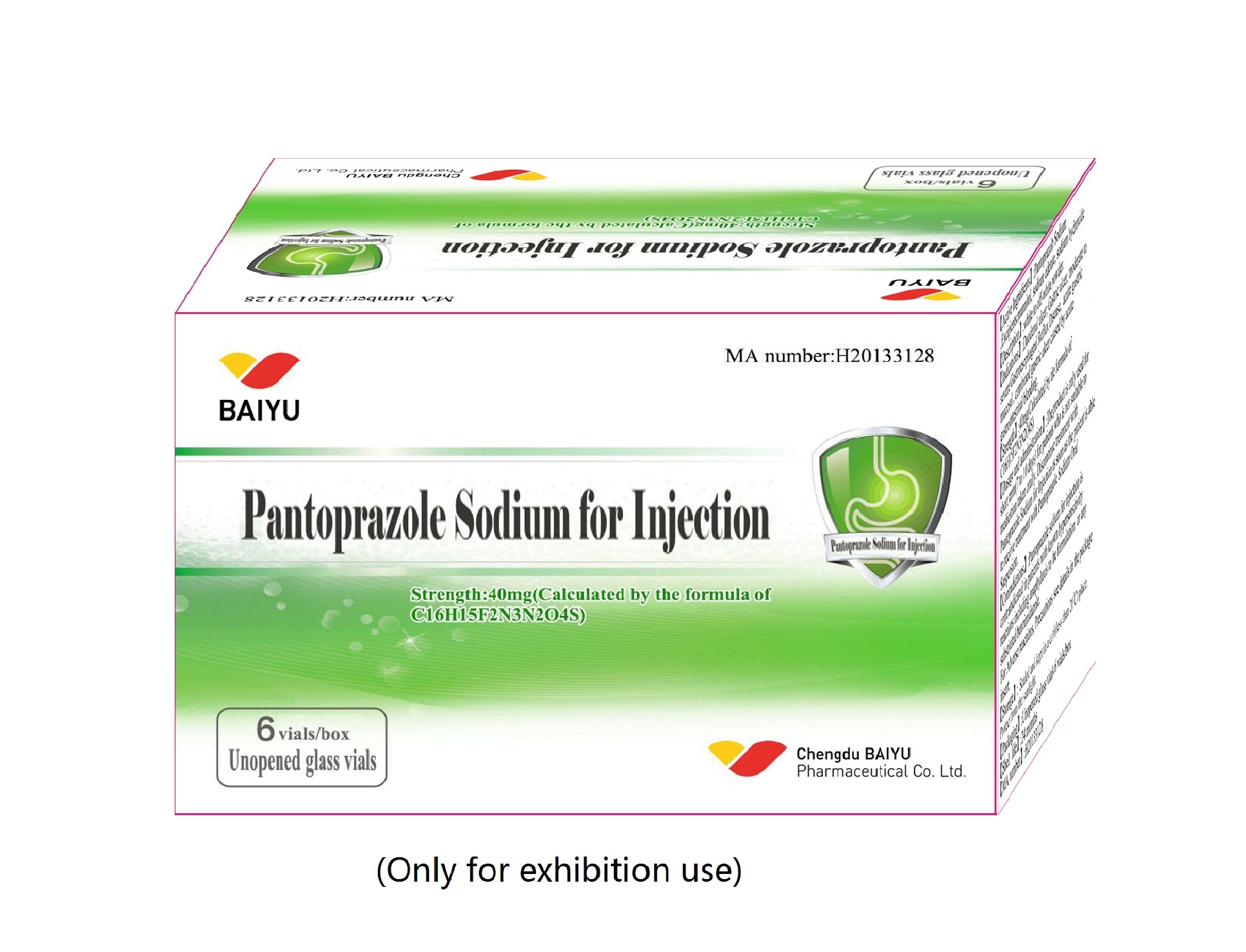
Pantoprazole sodium for injection
Generic name:Pantoprazole Sodium for Injection
Chemical name:5-difluoromethoxy-2-[(3,4- dimethoxy-2- pyridyl)- methyl]- sulfinyl-1H- bendimidazole sodium hydrate
Chemical structure:Molecular formula:C16H14F2N3NaO4S·H2O
Molecular weight:423.38
Active ingredient:Pantoprazole Sodium
Excipients:Mannitol, calcium edetate and sodium hydroxide
Description:White or off-white porous mass or (and) powder.
Indications:Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis, acute upper gastrointestinal bleeding caused by duodenal ulcer, gastrohelcosis, acute gastric mucosal lesion, complex gastrohelcosis, etc.
Strength:40mg;80mg (on the basis of C16H14F2N3NaO4S)
Dosage and administration
Pantoprazole sodium for injection is only a short-term (usually no more than 7 to 10 days) treatment for patients not suitable for oral medication. Once the patient can take the drug orally,do not continue to use it.
(1)Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis, acute upper gastrointestinal bleeding caused by duodenal ulcer, gastrohelcosis, acute gastric mucosal lesion, and complexgastrohelcosis: once 40mg to 80mg, 1 to 2 times a day. Prior to use, add 10ml of 0.9% sodium chloride injection into the vial to dissolve the freeze-dried content completely, then be added into 100 to 250ml of 0.9% sodium chloride injection for dilution, next followed by the intravenous infusion.The whole intravenous infusion period should be controlled within 15 to 60 minutes.
(2)Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis: once 40mg, once a day. Prior to use, add 10ml of 0.9% sodium chloride injection into the vial to dissolve the content completely, the dissolved solution can be used directly by intravenous infusion with a whole infusion period more than 2 minutes, or be added into a 100ml of 0.9% sodium chloride injection for dilution, then followed by the intravenous infusion. The whole infusion period should be controlled more than 15 minutes.
After the product is dissolved and diluted, it must be used within 4 hours. Do not use other solvents or other drugs to dissolve and dilute.
Adverse reaction
The common adverse reactions of pantoprazole sodium for injection are headache, diarrhea, nausea, abdominal pain, abdominal distention, vomiting, dizziness, and joint pain.
Other adverse reactions include the following:
Systemic: allergic reactions (including anaphylactic shock), fever, photosensitivity reactions, facial edema, peripheral edema, thrombophlebitis (intravenous injection only).
Gastrointestinal: constipation, dry mouth and hepatitis.
Blood system: leukopenia, thrombocytopenia.
Metabolism/nutrition: elevated creatine kinase (CPK), systemic edema, elevated triglycerides, elevated aminotransferases.
Musculoskeletal system: muscle soreness.
Central nervous system: depression, dizziness.
Skin and appendages: urticaria, rash, itching.
Special feeling: blurred vision.
Medication experience of the same kind drug on the market showed thatthe following adverse reactions have been confirmed.
Systemic disorders and medication site conditions: fatigue, fatigue, fatigue, weight changes.
Diseases of the immune system: Allergic reactions (including anaphylactic shock).
Diseases of the skin and subcutaneous tissues: severe skin reactions (some fatal), including erythema multiforme, malignant Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN) andangioneuroticedema.
Muscle strain: rhabdomyolysis, fractures.
Kidney and urinary system diseases: changes in renal function, interstitial nephritis.
Hepatobiliary disease: jaundice and liver failure caused by liver cell damage.
Mental disorders: hallucinations, confusion, insomnia, drowsiness.
Metabolic and nutritional disorders: hyponatremia, hypomagnesemia.
Contraindication
People who are allergic to this product or benzimidazole are prohibited from using this drug.
Packaging
Unopened glass vials, 10 vials/box
Approval number
GUOYAOZHUNZI H20143128
Chemical name:5-difluoromethoxy-2-[(3,4- dimethoxy-2- pyridyl)- methyl]- sulfinyl-1H- bendimidazole sodium hydrate
Chemical structure:Molecular formula:C16H14F2N3NaO4S·H2O
Molecular weight:423.38
Active ingredient:Pantoprazole Sodium
Excipients:Mannitol, calcium edetate and sodium hydroxide
Description:White or off-white porous mass or (and) powder.
Indications:Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis, acute upper gastrointestinal bleeding caused by duodenal ulcer, gastrohelcosis, acute gastric mucosal lesion, complex gastrohelcosis, etc.
Strength:40mg;80mg (on the basis of C16H14F2N3NaO4S)
Dosage and administration
Pantoprazole sodium for injection is only a short-term (usually no more than 7 to 10 days) treatment for patients not suitable for oral medication. Once the patient can take the drug orally,do not continue to use it.
(1)Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis, acute upper gastrointestinal bleeding caused by duodenal ulcer, gastrohelcosis, acute gastric mucosal lesion, and complexgastrohelcosis: once 40mg to 80mg, 1 to 2 times a day. Prior to use, add 10ml of 0.9% sodium chloride injection into the vial to dissolve the freeze-dried content completely, then be added into 100 to 250ml of 0.9% sodium chloride injection for dilution, next followed by the intravenous infusion.The whole intravenous infusion period should be controlled within 15 to 60 minutes.
(2)Duodenal ulcer, gastrohelcosis, moderate and severe reflux esophagitis: once 40mg, once a day. Prior to use, add 10ml of 0.9% sodium chloride injection into the vial to dissolve the content completely, the dissolved solution can be used directly by intravenous infusion with a whole infusion period more than 2 minutes, or be added into a 100ml of 0.9% sodium chloride injection for dilution, then followed by the intravenous infusion. The whole infusion period should be controlled more than 15 minutes.
After the product is dissolved and diluted, it must be used within 4 hours. Do not use other solvents or other drugs to dissolve and dilute.
Adverse reaction
The common adverse reactions of pantoprazole sodium for injection are headache, diarrhea, nausea, abdominal pain, abdominal distention, vomiting, dizziness, and joint pain.
Other adverse reactions include the following:
Systemic: allergic reactions (including anaphylactic shock), fever, photosensitivity reactions, facial edema, peripheral edema, thrombophlebitis (intravenous injection only).
Gastrointestinal: constipation, dry mouth and hepatitis.
Blood system: leukopenia, thrombocytopenia.
Metabolism/nutrition: elevated creatine kinase (CPK), systemic edema, elevated triglycerides, elevated aminotransferases.
Musculoskeletal system: muscle soreness.
Central nervous system: depression, dizziness.
Skin and appendages: urticaria, rash, itching.
Special feeling: blurred vision.
Medication experience of the same kind drug on the market showed thatthe following adverse reactions have been confirmed.
Systemic disorders and medication site conditions: fatigue, fatigue, fatigue, weight changes.
Diseases of the immune system: Allergic reactions (including anaphylactic shock).
Diseases of the skin and subcutaneous tissues: severe skin reactions (some fatal), including erythema multiforme, malignant Stevens-Johnson syndrome, toxic epidermal necrolysis (TEN) andangioneuroticedema.
Muscle strain: rhabdomyolysis, fractures.
Kidney and urinary system diseases: changes in renal function, interstitial nephritis.
Hepatobiliary disease: jaundice and liver failure caused by liver cell damage.
Mental disorders: hallucinations, confusion, insomnia, drowsiness.
Metabolic and nutritional disorders: hyponatremia, hypomagnesemia.
Contraindication
People who are allergic to this product or benzimidazole are prohibited from using this drug.
Packaging
Unopened glass vials, 10 vials/box
Approval number
GUOYAOZHUNZI H20143128